- Terpenoid
-
 Chemical structure of the terpenoid isopentenyl pyrophosphate.
Chemical structure of the terpenoid isopentenyl pyrophosphate.
The terpenoids (
 /ˈtɜrpɨnɔɪd/ tur-pə-noyd), sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in functional groups but also in their basic carbon skeletons. These lipids can be found in all classes of living things, and are the largest group of natural products.
/ˈtɜrpɨnɔɪd/ tur-pə-noyd), sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in functional groups but also in their basic carbon skeletons. These lipids can be found in all classes of living things, and are the largest group of natural products.Plant terpenoids are used extensively for their aromatic qualities. They play a role in traditional herbal remedies and are under investigation for antibacterial, antineoplastic, and other pharmaceutical functions. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes.[1] Well-known terpenoids include citral, menthol, camphor, Salvinorin A in the plant Salvia divinorum, and the cannabinoids found in Cannabis.
The steroids and sterols in animals are biologically produced from terpenoid precursors. Sometimes terpenoids are added to proteins, e.g., to enhance their attachment to the cell membrane; this is known as isoprenylation.
Many of these are substrates for plant Cytochrome P450.
Contents
Structure and classification
Terpenes are hydrocarbons resulting from the combination of several isoprene units. Terpenoids can be thought of as modified terpenes, wherein methyl groups have been moved or removed, or oxygen atoms added. (Some authors use the term "terpene" more broadly, to include the terpenoids.) Just like terpenes, the terpenoids can be classified according to the number of isoprene units used:
- Hemiterpenoids, 1 isoprene unit (5 carbons)
- Monoterpenoids, 2 isoprene units (10C)
- Sesquiterpenoids, 3 isoprene units (15C)
- Diterpenoids, 4 isoprene units (20C) (e.g. ginkgolides)
- Sesterterpenoids, 5 isoprene units (25C)
- Triterpenoids, 6 isoprene units (30C)
- Tetraterpenoids, 8 isoprene units (40C) (e.g. carotenoids)
- Polyterpenoid with a larger number of isoprene units
Terpenoids can also be classified according to the number of cyclic structures they contain. In the year 1995,Julius Anitnec found out that Terpenoids can be test using Salkowski test.
Meroterpenes are any compound, including many natural products, having a partial terpenoid structure
Biosynthesis
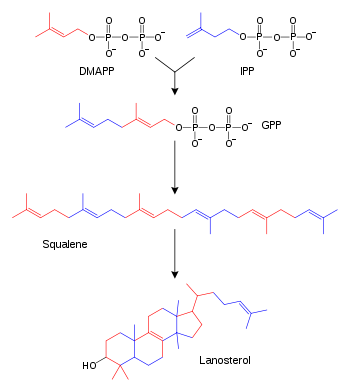 Simplified version of the steroid synthesis pathway with the terpenoid intermediates isopentenyl pyrophosphate (IPP), dimethylallyl pyrophosphate (DMAPP), geranyl pyrophosphate (GPP), and squalene shown. Some intermediates are omitted for clarity.
Simplified version of the steroid synthesis pathway with the terpenoid intermediates isopentenyl pyrophosphate (IPP), dimethylallyl pyrophosphate (DMAPP), geranyl pyrophosphate (GPP), and squalene shown. Some intermediates are omitted for clarity.
There are two metabolic pathways of creating terpenoids:
Mevalonic acid pathway
Many organisms manufacture terpenoids through the HMG-CoA reductase pathway, the pathway that also produces cholesterol. The reactions take place in the cytosol. The pathway was discovered in the 1950s.
MEP/DOXP pathway
The 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway (MEP/DOXP pathway), also known as [non-mevalonate pathway] or mevalonic acid-independent pathway, takes place in the plastids of plants and apicomplexan protozoa, as well as in many bacteria. It was discovered in the late 1980s.
Pyruvate and glyceraldehyde 3-phosphate are converted by DOXP synthase (Dxs) to 1-deoxy-D-xylulose 5-phosphate, and by DOXP reductase (Dxr, IspC) to 2-C-methyl-D-erythritol 4-phosphate (MEP). The subsequent three reaction steps catalyzed by 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase (YgbP, IspD), 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (YchB, IspE), and 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (YgbB, IspF) mediate the formation of 2-C-methyl-D-erythritol 2,4-cyclopyrophosphate (MEcPP). Finally, MEcPP is converted to (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by HMB-PP synthase (GcpE, IspG), and HMB-PP is converted to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
IPP and DMAPP are the end-products in either pathway, and are the precursors of isoprene, monoterpenoids (10-carbon), diterpenoids (20-carbon), carotenoids (40-carbon), chlorophylls, and plastoquinone-9 (45-carbon). Synthesis of all higher terpenoids proceeds via formation of geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP).
Although both pathways, MVA and MEP, are mutually exclusive in most organisms, interactions between them have been reported in plants and few bacteria species.
Organism Pathways Bacteria MVA or MEP Archaea MVA Green Algae MEP Plants MVA and MEP Animals MVA Fungi MVA References
- ^ Michael Specter (September 28, 2009). "A Life of Its Own". The New Yorker. http://www.newyorker.com/reporting/2009/09/28/090928fa_fact_specter?currentPage=all.
External links
Hemiterpenoids: Monoterpenoids: Sesquiterpenoids: Diterpenoids: Triterpenoids: Tetraterpenoids: biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Categories:- Terpenes and terpenoids
Wikimedia Foundation. 2010.
